Abstract
Background: Revised international prognostic score for Waldenstrom macroglobulinemia (rIPSSWM) has been recently validated for symptomatic WM patients in need of treatment and was built on the independent prognostic role of Age, LDH, Serum Albumin and Beta2microglobulin. In 2010 the NF10 study was started by the Fondazione Italiana Linfomi as a prospective registry specifically devised for investigating the prognosis of, Indolent non follicular B-Cell Lymphomas (INFL) including small lymphocytic lymphoma (SLL), lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia (LPL/WM) and marginal zone lymphomas (MZL).
Methods: We aimed to assess the prognostic role of RIPSSWM (ref) on an unselected population of treatment naïve patients with LPL/WM enrolled in the NF10 registry. Main study endpoint was Overall Survival. Secondary endpoint was treatment free survival (TFS).
Results: From July 2010 to January 2020, 1535 INFL cases were registered and 1328 validated. Three hundred and seventeen cases were LPL/WM. Median age was 69 years (range 60-76); 6% had ECOG performance status > 1, median hemoglobin concentration was 12 g/dl (IQR 10 - 13.6), median serum albumin was 4 g/dl (IQR 3.6 - 4.3); serum CM was present in 254 patients with median concentration of 1.6 g/dl (IQR 0.74 - 2.4). Lactate dehydrogenase and b2-microglobulin were elevated in 21% and 59% of cases, respectively.
Overall, 191 patient received systemic therapy. Immediate systemic therapy was planned in 133 (42%) patients. Additionally 58 patients required systemic treatment due to progressive disease from watch and wait. Median time to treatment for WW patients was 18 months (95% CI 15 to 28). When systemic therapy was prescribed rituximab (R) was used in 82% and was combined with cytotoxic therapy in 76% of cases. Regarding immunochemotherapy regimens, R was combined with bendamustine in 34%, alkylating agents in 30%, fludarabine in 8%, and CHOP like in 4%.
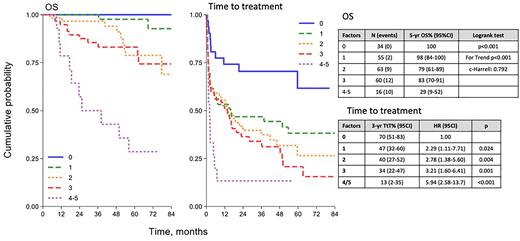
With 58 months of median follow up (1 - 114), 5-year overall survival (OS) was 83% (95%CI: 78-88). In univariate analysis of OS, Age (>75years), elevated LDH, elevated B2M, and low serum albumin (<3.5) were associated with a different risk of death; the initial choice of deferring therapy did not impact on OS. Revised IPSSWM score could be calculated for 228 patients: 34(15%), 55(24%), 63(28%), 60(26%), and 16(7%) patients had 0,1,2,3, or 4-5 risk factors, respectively. Five-year OS was 100%, 98%, 79%, 83%, and 29% by risk group (p<0.001; c-Harrel 0.792: Figure1). The risk of dying due to lymphoma progression or due to other causes was similarly low for low risk patients (0-1 risk factors). Intermediate (2-3 RF) and high risk patients (4-5 RF) had a three-fold higher risk of dying due to causes unrelated to lymphoma compared to lymphoma related deaths. rIPSSWM was also correlated with a different TFS among patients initially observed (Figure 1: median TFS not reached for 0 RF, vs 15 months for 1-3 RF, vs 1.5 months for 4-5 RF p <0.001).
Conclusions: We were able to validate the prognostic role of rIPSSWM in an unselected population of newly diagnosed patients with LPL/WM thus extending the usefulness of this score to asymptomatic patients. The rIPSSWM was particularly able to identify a small group of patients at very high risk of death within few months from the diagnosis. Moreover the rIPSSWM was also able to predict the time to initial treatment for patients who were initially observed. The NF10 study confirms that a web-based world-wide cooperation allows the collection of a relevant and complete data set, providing a platform for future prognostic and pathobiological studies.
Disclosures
Ferrero:Incyte: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; Gilead: Research Funding; Gentili: Speakers Bureau; Clinigen: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jannsen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Servier: Honoraria, Speakers Bureau. Ferreri:Ospedale San Raffaele srl: Patents & Royalties: NGR-hTNAF/RCHOP in relapsed/refractory PCNSL; SNGR-hTNF in brain tumours; Adienne: Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; PletixaPharm: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Genmab: Research Funding; ADC Therapeutics: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Amgen: Research Funding; Pharmacyclics: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Hutchison Medipharma: Research Funding; Beigene: Research Funding. Arcaini:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees. Luminari:GENMAB: Membership on an entity's Board of Directors or advisory committees; TAKEDA: Membership on an entity's Board of Directors or advisory committees; ROCHE: Membership on an entity's Board of Directors or advisory committees; Jannsen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Regeneron: Membership on an entity's Board of Directors or advisory committees. Varettoni:ABBVIE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; JANNSEN: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal